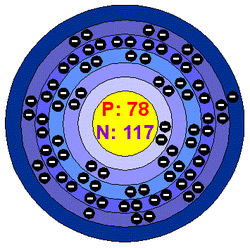
Platinum is a chemical element with the symbol Pt and an atomic number of 78.
Its name is derived from the Spanish term platina, which is literally translated into "little silver". It is a dense, malleable, ductile, precious, gray-white transition metal.
Its name is derived from the Spanish term platina, which is literally translated into "little silver". It is a dense, malleable, ductile, precious, gray-white transition metal.
Platinum is widely used as a catalyst converting methyl alcohol vapors (CH4O) into formaldehyde (CH2O) on contact.It glows red hot in the process. This effect is used to make small hand warmers. Platinum is also used in a catalytic converter, a device found in the exhaust systems of most cars. Catalytic converters combine carbon monoxide (CO) and unburned fuel from a car's exhaust with oxygen from the air, forming carbon dioxide (CO2) and water vapor (H2O). Platinum is also used as a catalyst in the production of sulfuric acid (H2SO4) and in the cracking of petroleum products. Fuel cells, devices that combine hydrogen and oxygen to produce electricity and water, also use platinum as a catalyst
Platinum has a melting point of 1772 °C, boiling point of 3827 +/- 100 °C, specific gravity of 21.45 (20 °C), with a valence of 1, 2, 3, or 4. Platinum is a ductile and malleable silvery-white metal. It does not oxidize in air at any temperature, although it is corroded by cyanides, halogens, sulfur, and caustic alkalis. Platinum does not dissolve in hydrochloric or nitric acid, but will dissolve when the two acids are mixed to form aqua regia.
Platinum is used in jewelry, wire, to make crucibles and vessels for laboratory work, electrical contacts, thermocouples, for coating items that must be exposed to high temperatures for long periods of time or must resist corrosion, and in dentistry. Platinum-cobalt alloys have interesting magnetic properties. Platinum absorbs large amounts of hydrogen at room temperature, yielding it at red heat. The metal is often used as a catalyst. Platinum wire will glow red-hot in the vapor of methanol, where is acts as a catalyst, converting it for formaldyhde. Hydrogen and oxygen will explode in the presence of platinum.
Platinum is used in jewelry, wire, to make crucibles and vessels for laboratory work, electrical contacts, thermocouples, for coating items that must be exposed to high temperatures for long periods of time or must resist corrosion, and in dentistry. Platinum-cobalt alloys have interesting magnetic properties. Platinum absorbs large amounts of hydrogen at room temperature, yielding it at red heat. The metal is often used as a catalyst. Platinum wire will glow red-hot in the vapor of methanol, where is acts as a catalyst, converting it for formaldyhde. Hydrogen and oxygen will explode in the presence of platinum.

 RSS Feed
RSS Feed