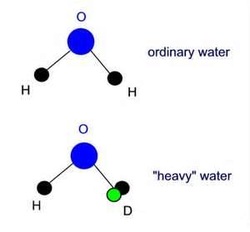
Heavy water,also known as deuterium oxide or 2H2O or D2O, is a form of water that contains a higher than normal amount of the hydrogen isotopedeuterium, (also known as "heavy hydrogen") rather than the common hydrogen-1 isotope that makes up most of the hydrogen in normal water.
Some or most of the hydrogen atoms in heavy water contain an extra neutron, causing each hydrogen atom to be twice as heavy as a normal hydrogen atom. The increased weight of the hydrogen in the water thus makes it slightly more dense. The colloquial term heavy water is often also used to refer a highly enriched water mixture that contains mostly deuterium oxide but also contains some ordinary water molecules as well
Some or most of the hydrogen atoms in heavy water contain an extra neutron, causing each hydrogen atom to be twice as heavy as a normal hydrogen atom. The increased weight of the hydrogen in the water thus makes it slightly more dense. The colloquial term heavy water is often also used to refer a highly enriched water mixture that contains mostly deuterium oxide but also contains some ordinary water molecules as well
Heavy water is not radioactive. The density of heavy water, in its pure form, is 11% grater than water but is physically and chemically similar. This difference in density is larger than in any other commonly occurring isotope-substituted compound because deuterium is unique among heavy stable isotopes in being twice as heavy as the lightest isotope.This difference increases the strength of water's hydrogen-oxygen bonds, and in turn is enough to cause differences thats needed for some biochemical reactions. The human body naturally contains deuterium equivalent to five grams of heavy water, which is harmless. When a large fraction of water (> 50%) in higher organisms is replaced by heavy water, the result is cell dysfunction and death.
 RSS Feed
RSS Feed